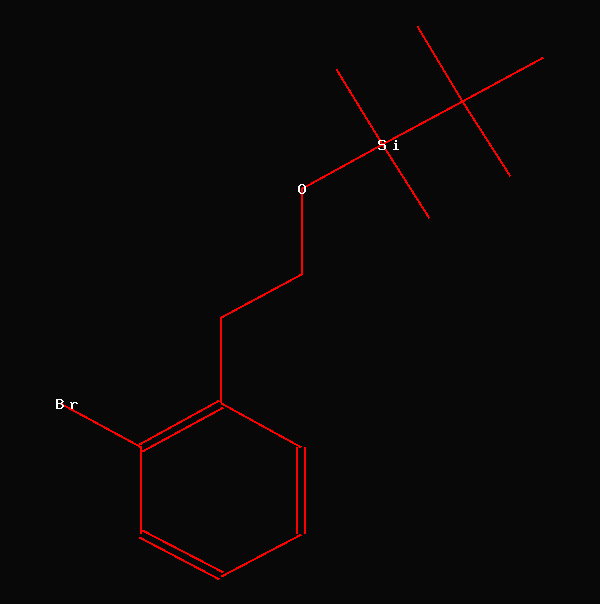
NMR-Data from Canadian Journal of
Chemistry, 77, 146 (1999)
This compound has been synthesized and
the analytical data have been published in the above mentioned article.
The tert.-butyldimethylsilyl group is a frequently occuring protecting
group in organic chemistry. A CAS-search using SCIFINDER revealed
207,820 structures, when using this structural fragment as query.
A prediction of the carbon chemical
shiftvalues for this protecting group leads to values of -5ppm for
the Si-CH3 group, 18ppm for the quarternary carbon and 25ppm
for the -CH3 in
the tBu-group. (Values rounded, nearly identical values
obtained by HOSE and NN calculation using CSEARCH)
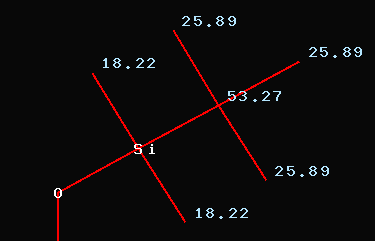
When looking into the above mentioned
article (Compound #8) we find the following values for the protecting
group: 53.27 (SiC(CH3)3),
25.89 (SiC(CH3)3),
18.22
(Si(CH3)2)
Carbon
position
|
CSEARCH-HOSE
|
CSEARCH-NN
|
Literature
|
SiC(CH3)3
|
18.3
|
18.5
|
53.27
ppm
|
SiC(CH3)3
|
25.9
|
25.2
|
25.89
ppm
|
Si(CH3)2
|
-5.5
|
-5.6
|
18.22
ppm
|
Obviously there is some misassignment
when looking at the signals at 18.22ppm and 25.89ppm respectively. The
open question is the remaining signal at 53.27ppm. The only explanation
I can offer is - when reading the experimental part carefully -
dichloromethane, which was used during the last step of workup.
Within the article there is no
numbering scheme for the compounds given - in such a case the
naumbering from the nomenclature is usually applied. In this particular
case the C-2 (138.29ppm) and C-1 (124.56ppm) [the two aromatic signals
which are clearly assigned] should also be reversed - maybe the term
'C-ipso' is with respect to the bromine substitution. At least the data
representation is not very 'user-friendly' in the case of the aromatic
carbons, whereas the signals of the prtecting group are definitely
wrong.
Page
written by: Wolfgang.Robien(-at-)c13nmr.at
Page online since: March 4th,
2008